(Go to Shell Correction Plots)
Shell Corrections and Mean Ionization Potentials are used in Bethe-Bloch stopping for high velocity ions. In SRIM, these ions are considered to have energies above 1 MeV/amu. For a complete explanation of Shell Corrections and Mean Ionization Potentials, see "Stopping of Energetic Light Ions in Elemental Matter", J. F. Ziegler, Applied Physics Reviews / J. Applied Physics, 85, 1249-1272 (1999). This paper is included as an Acrobat PDF file (600 kB). (Download Paper)
Shown below is the normal expansion of the Bethe Bloch equation. The two principle terms are the "Mean Ionization Energy", noted as <I> in equation (10), and "C/Z2" which is called the "Shell Corection". Basically, the Mean Ionization Potential, <I>, is the averaged excitation potential per electron in the target. The theoretical calculation of <I> has a long history, for it is a straightforward calculation which may be done with almost any theoretical atom. Summaries can be found in several reviews [1-4]
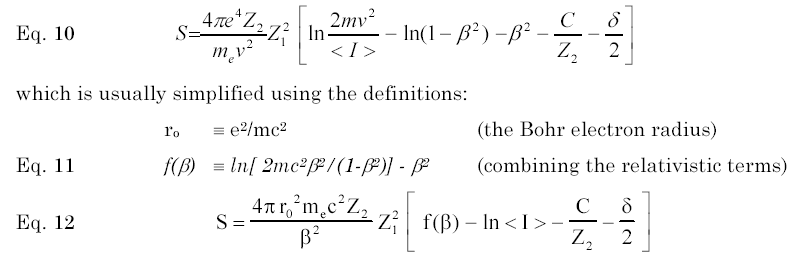
Shell corrections (usually noted using the symbol C/Z2) constitute a large correction to proton stopping powers in the energy range of 1-100 MeV, with a maximum correction of about 6%. It corrects the Bethe-Bloch theory requirement that the particle’s velocity is far greater than the bound electron velocity. As a particle velocity decreases from relativistic energies, the particle-electron collisions need to be considered with detailed evaluation of each target electron’s orbital bonding in order to obtain accurate stopping powers. Many authors have contributed to the theoretical definition of non-relativistic shell corrections, see discussions noted below.[5-10].
Fano suggested [1] that the calculation of the mean ionization potential, and the shell correction, could properly be linked as a single term which could be evaluated directly from experimental stopping data. This approach has the advantage of isolating the two factors in the Bethe-Bloch equation which require extensive theoretical models, i.e. <I>, and C/Z2. Using this equation, experimental data may be shown in reduced form and compared to theoretical calculations.
The importance of this approach is for the interpolation of stopping powers to targets with little experimental data. If the summed terms could be directly obtained from experimental data, then these can be used to interpolate for stopping powers of similar targets without experimental data. This technique was first used by Ziegler to extract the summed correction terms in order to normalize stopping calculations for targets without data, or to extrapolate to energies without experimental data. [11]
1 U. Fano, Chr., Studies in Penetration of Charged Particles in Matter, Nucl. Sci. Rpt.. 39, U. S. National Academy of Sciences, Washington, 1-338 (1964).
2 J. F. Ziegler, “Handbook of Stopping Cross-Sections for Energetic Ions in All Elements”, Pergamon Press (1980).
3 S. P. Ahlen, Rev. Mod. Phys., vol. 52, 121 (1980).
4 ICRU-49, H. O. Wyckoff (ICRU Scientific Counsellor), “Stopping Powers and Ranges for Protons and Alpha Particles”, Intl. Comm. on Rad. Units, Bethseda, MD (1993).
5 N. Bohr, Kgl. Dansk. Vid. Sel., Mat.-Fys. Medd., 18, 1 (1948).
6 M. C. Walske, Phys. Rev., 88, 1283 (1952), and Phys. Rev., 101, 940 (1956).
7 M. C. Walske, Phys. Rev., 101, 940 (1956).
8 G. S. Khandelwal, Nucl. Phys., A116, 97 (1968).
9 H. Bichsel, Univ. of Calif Rpt. USC-136-120.
10 G. S. Khandelwal and E. Merzbacher, Phys. Rev., 144, 349 (1966).
11 J. F. Ziegler, “Handbook of Stopping Cross-Sections for Energetic Ions in All Elements”, Pergamon Press (1980).
![]()
The plot ordinate is the sum of the two terms, the mean ionization potential, <I>, and the shell correction, C/Z2.
The plot abscissa is in ion energy units of MeV/amu.
The data plotted are experimental stopping powers for H and He ions, reduced to [ln<I> + C/Z2] using the Bethe-Bloch theory. Experimental Values are in different colors, coded to brief descriptions of various papers noted in the upper right of the plot. For H ions, the plot-symbols for proton ions are "H", deuterium ions are"D" and helium ions are "He".
The solid blue line (with squares) is [ln<I> + C/Z2] as evaluated in SRIM.
The solid horizontal RED line is ln<I>, with its value noted below the line.
The difference between the RED line and the solid blue line is the shell correction term.
Citations for the experimental points are listed in the upper right corner of the plot. The Citation Number is from the database to be published on the CD-ROM. The number is the year of publication and the four letters are from the name of the first author. Also shown are the number of data points plotted from each paper. These are divided into two groups, those for energies below 1 MeV/u and those above this energy. These details plus the data color should help to correlate plotted data with citation.
At the bottom of each plot is a line indicating the magnitude of a 1% effect on the final stopping power. This means that a correction of the value between this line and the abscissa axis will lead to a change of 1% of stopping. Note that for low energies, a very small correction leads to a 1% change, while at higher energies the same correction has less effect.
Note: The plots on this website are low resolution for efficiency.
For high-resolution plots, contact the author.
TargetAtomicNumber |
TargetAtomicSymbol |
TargetElementName |
Plots |
| 01 | H | Hydrogen | Plot |
| 02 | He | Helium | Plot |
| 03 | Li | Lithium | Plot |
| 04 | Be | Beryllium | Plot |
| 05 | B | Boron | Plot |
| 06 | C | Carbon | Plot |
| 07 | N | Nitrogen | Plot |
| 08 | O | Oxygen | Plot |
| 09 | F | Fluorine | Plot |
| 10 | Ne | Neon | Plot |
| 11 | Na | Sodium | Plot |
| 12 | Mg | Magnesium | Plot |
| 13 | Al | Aluminum | Plot |
| 14 | Si | Silicon | Plot |
| 15 | P | Phosphorus | Plot |
| 16 | S | Sulfur | Plot |
| 17 | Cl | Chlorine | Plot |
| 18 | Ar | Argon | Plot |
| 19 | K | Potassium | Plot |
| 20 | Ca | Calcium | Plot |
| 21 | Sc | Scandium | Plot |
| 22 | Ti | Titanium | Plot |
| 23 | V | Vanadium | Plot |
| 24 | Cr | Chromium | Plot |
| 25 | Mn | Manganese | Plot |
| 26 | Fe | Iron | Plot |
| 27 | Co | Cobalt | Plot |
| 28 | Ni | Nickel | Plot |
| 29 | Cu | Copper | Plot |
| 30 | Zn | Zinc | Plot |
| 31 | Ga | Gallium | Plot |
| 32 | Ge | Germanium | Plot |
| 33 | As | Arsenic | Plot |
| 34 | Se | Selenium | Plot |
| 35 | Br | Bromine | Plot |
| 36 | Kr | Krypton | Plot |
| 37 | Ru | Rubidium | Plot |
| 38 | Sr | Strontium | Plot |
| 39 | Y | Yttrium | Plot |
| 40 | Zr | Zirconium | Plot |
| 41 | Nb | Niobium | Plot |
| 42 | Mo | Molybdenum | Plot |
| 43 | Tc | Technetium | Plot |
| 44 | Ru | Ruthenium | Plot |
| 45 | Rh | Rhodium | Plot |
| 46 | Pd | Palladium | Plot |
| 47 | Ag | Silver | Plot |
TargetAtomicNumber |
TargetAtomicSymbol |
TargetElementName |
Plots |
| 48 | Cd | Cadmium | Plot |
| 49 | In | Indium | Plot |
| 50 | Sn | Tin | Plot |
| 51 | Sb | Antimony | Plot |
| 52 | Te | Tellurium | Plot |
| 53 | I | Iodine | Plot |
| 54 | Xe | Xenon | Plot |
| 55 | Cs | Cesium | Plot |
| 56 | Ba | Barium | Plot |
| 57 | La | Lanthanum | Plot |
| 58 | Ce | Cerium | Plot |
| 59 | Pr | Praseodymium | Plot |
| 60 | Nd | Neodymium | Plot |
| 61 | Pm | Promethium | Plot |
| 62 | Sm | Samarium | Plot |
| 63 | Eu | Europium | Plot |
| 64 | Gd | Gadolinium | Plot |
| 65 | Tb | Terbium | Plot |
| 66 | Dy | Dysprosium | Plot |
| 67 | Ho | Holium | Plot |
| 68 | Er | Erbium | Plot |
| 69 | Tm | Thulium | Plot |
| 70 | Yb | Ytterbium | Plot |
| 71 | Lu | Lutetium | Plot |
| 72 | Hf | Hafnium | Plot |
| 73 | Ta | Tantalum | Plot |
| 74 | W | Tungsten | Plot |
| 75 | Re | Rhenium | Plot |
| 76 | Os | Osmium | Plot |
| 77 | Ir | Iridium | Plot |
| 78 | Pt | Platinum | Plot |
| 79 | Au | Gold | Plot |
| 80 | Hg | Mercury | Plot |
| 81 | Tl | Thallium | Plot |
| 82 | Pb | Lead | Plot |
| 83 | Bi | Bismuth | Plot |
| 84 | Po | Polonium | Plot |
| 85 | At | Asatine | Plot |
| 86 | Rn | Radon | Plot |
| 87 | Fr | Francium | Plot |
| 88 | Ra | Radium | Plot |
| 89 | Ac | Actinium | Plot |
| 90 | Th | Thorium | Plot |
| 91 | Pa | Proactinium | Plot |
| 92 | U | Uranium | Plot |
TargetAtomicNumber |
TargetAtomicSymbol |
TargetElementName |
Plots |
Note: The plots on this website have relatively low resolution for efficiency.
For high-resolution plots, contact the author.
![]()